Our Technology
Retained Display TM (ReD) System – Scalable highthroughput and high-precision pHLA antibody engineering platform.
Myrio’s engine of innovation, the RedTM system generates diverse human antibody panels with single residue pHLA cancer-targeting resolution, engineered with compatibility across major HLA variants, enabling cancer patients across populations potent and effective immuno-oncology therapies.
ReDTM is the only platform that generates cancer-specific antibodies for the all of the major HLA types. Additionally, Myrio has pioneered the ability to ‘break restriction’ – generating antibodies which can target a peptide presented by more than one HLA, and thus increase the addressable patient population.
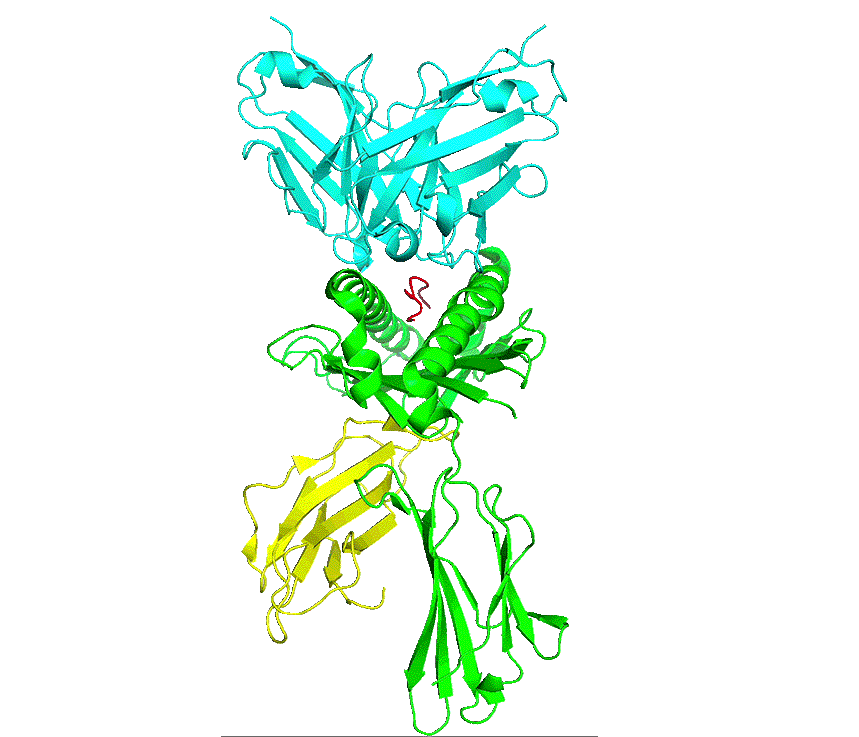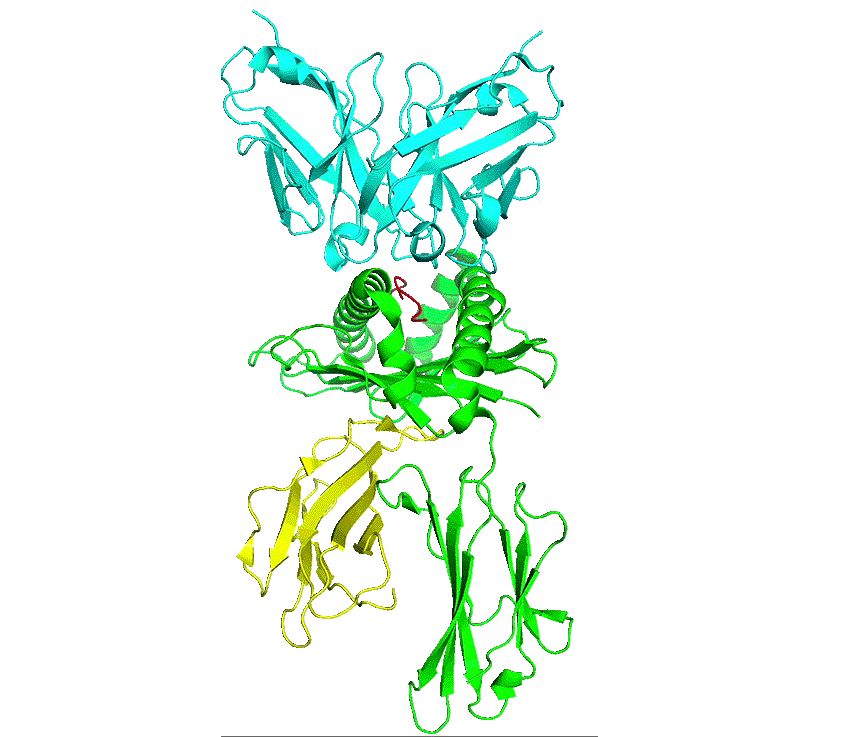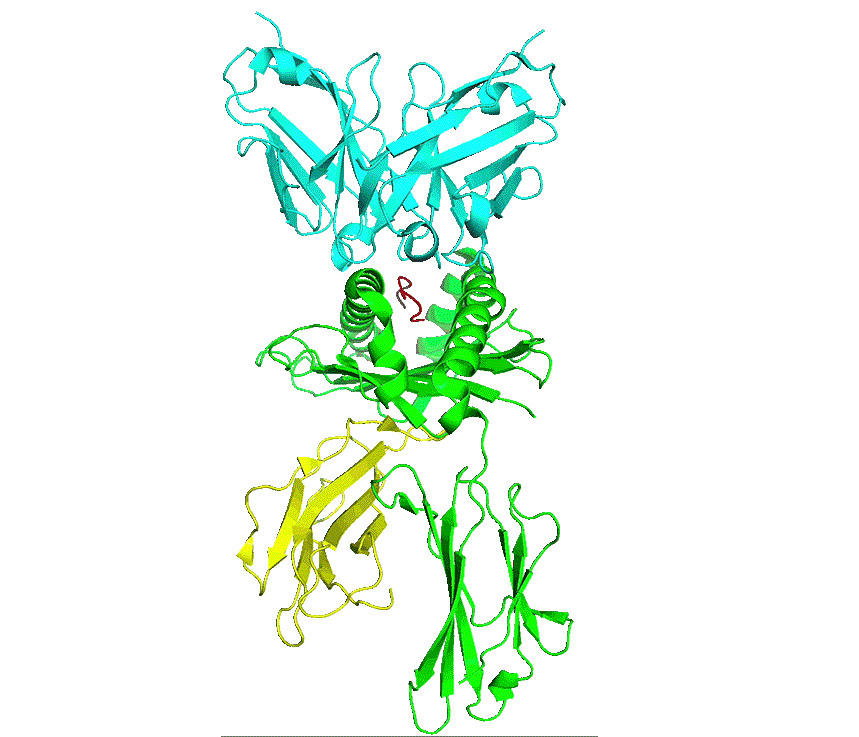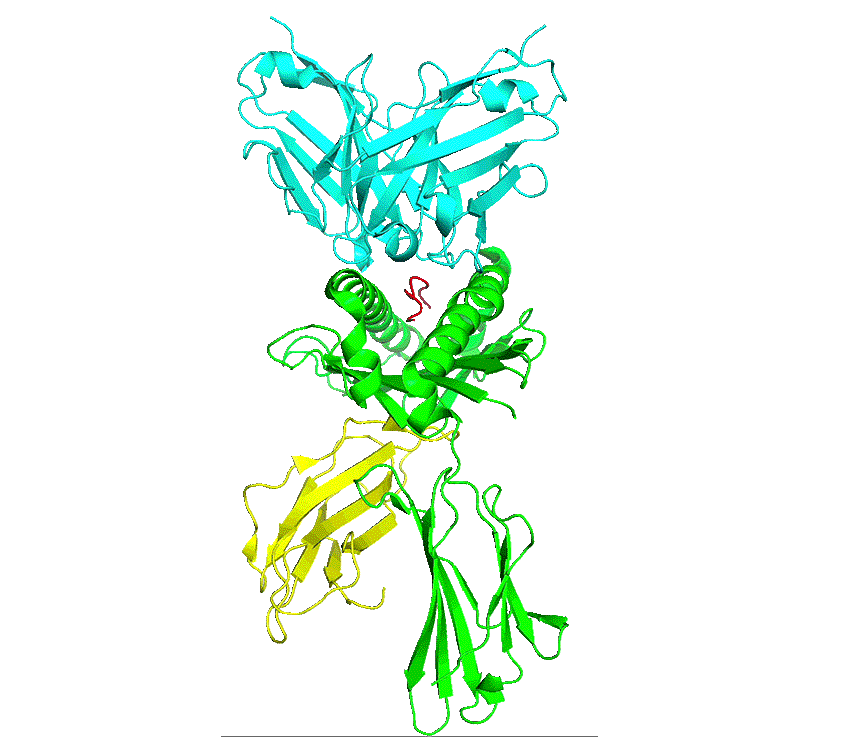
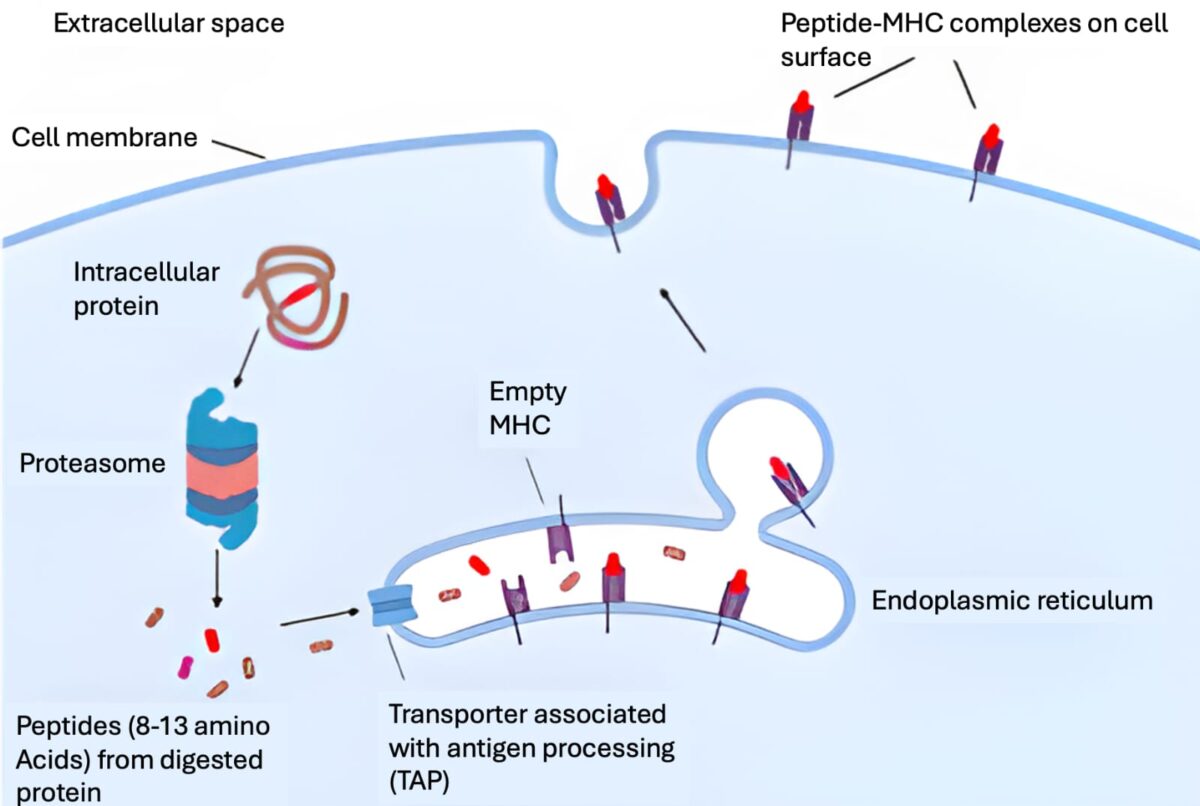
pHLA complex
Sensitive and discrete differentiation between healthy and cancerous cells
As part of natural protein turnover, all proteins get processed by the proteasome into short peptides, which are then processed by the endoplasmic reticulum and loaded onto the major histocompatibility complex (MHC) – human leucocyte antigen (HLA) in humans. The peptide-HLA (pHLA) complex is transported to the cell surface providing intrinsic surveillance of the internal state of cells.
The natural binding partner for a peptide-HLA complex is the T-cell receptor which has to be able to discriminate between peptide-HLA complexes on the basis of specific interactions with the peptide. Thus, cells that are infected by a virus or contain an altered protein can be detected and destroyed by T-cells.
The challenge of targeting peptide-HLA complexes
Antibodies are similar to T-cell receptors and possess innate characteristics which make it advantageous for engineering and therapeutics. Developing therapeutic antibodies against pHLA complexes are extremely challenging, requiring consideration and optimisation of hundreds of interdependent characteristics and parameters, with antibodies requiring exquisite single-residue precision recognition of peptide sequences on the pHLA complex to delineate healthy from cancer states.
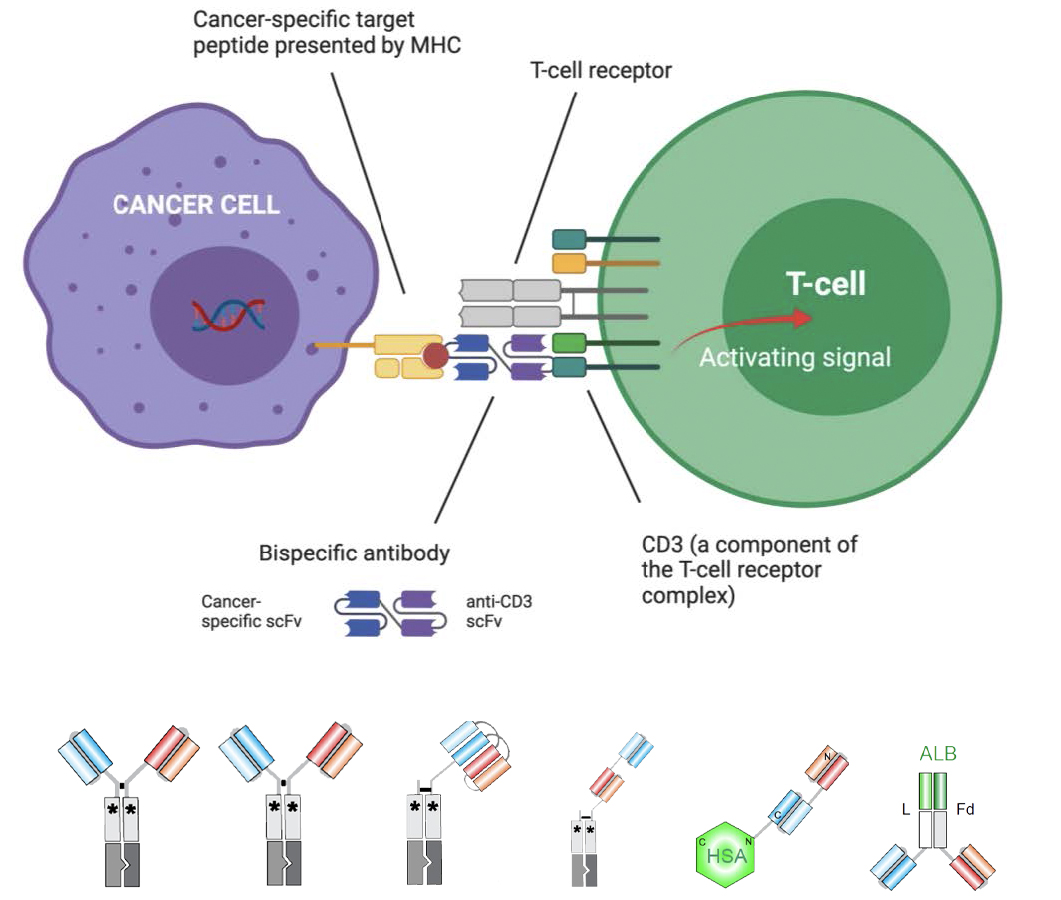
CAR-T and Bispecific Antibodies –Utilising pHLAcomplex for immuno-oncology therapeutics
While the natural engagers of pHLA complexes are T-cells and T-cell receptors, there are many targets for which it is difficult to develop good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity.
Antibodies are ideal therapeutic scaffolds – highly selective and generally have higher affinities than T-cell receptors. Immuno-oncology therapeutics are currently exploring two similar paradigms for leveraging pHLA complexes – Chimeric Antigen Receptor on T-cells (CAR-T) and Bispecific antibodies which bind to pHLA complexes and T-cell activating proteins such as CD3. By directing T-cell engagement and activation to cancer-specific peptides on the pHLA complex, precision cancer cell kills can be achieved.
ReDTM platform and Ruby library – Scalable, Flexible and High Precision Antibodies
Myrio constructed and operates the Retained Display (ReD) platform and Ruby mass diversity human scaffold library (> 300 billion clonal identities). Together these form an antibody engineering system that is designed to generate and develop pHLA discriminating antibodies.
ReD and Ruby employs lambdoid phage display, which demonstrate superior characteristics in:
- Higher avidity panning of libraries to ensure high diversity clonal capture against pHLA complexes.
- Lossless panning and enrichment of clones to separate affinity and avidity from clonal numbers.
- Speed and Sensitivity, with picomolar dissociation constant antibodies generated within 20 weeks.
- Human antibody backbone, ready for therapeutic engineering and format conversion.
- Libraries compatible with multiple HLA variants for coverage across patient geographies and demographies
pHLA complex – Sensitive and discrete differentiation between healthy and cancerous cells
As part of natural protein turnover, all proteins get processed by the proteasome into short peptides, which are then processed by the endoplasmic reticulum and loaded onto the major histocompatibility complex (MHC) - human leucocyte antigen (HLA) in humans. The peptide-HLA (pHLA) complex is transported to the cell surface providing intrinsic surveillance of the internal state of cells.
The natural binding partner for a peptide-HLA complex is the T-cell receptor which has to be able to discriminate between peptide-HLA complexes on the basis of specific interactions with the peptide. Thus, cells that are infected by a virus or contain an altered protein can be detected and destroyed by T-cells.
The challenge of targeting peptide-HLA complexes Antibodies are similar to T-cell receptors and possess innate characteristics which make it advantageous for engineering and therapeutics. Developing therapeutic antibodies against pHLA complexes are extremely challenging, requiring consideration and optimisation of hundreds of interdependent characteristics and parameters, with antibodies requiring exquisite singleresidue precision recognition of peptide sequences on the pHLA complex to delineate healthy from cancer states.
CAR-T and Bispecific Antibodies – Utilising pHLA complex for immuno-oncology therapeutics
While the natural engagers of pHLA complexes are T-cells and T-cell receptors, there are many targets for which it is difficult to develop good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity.
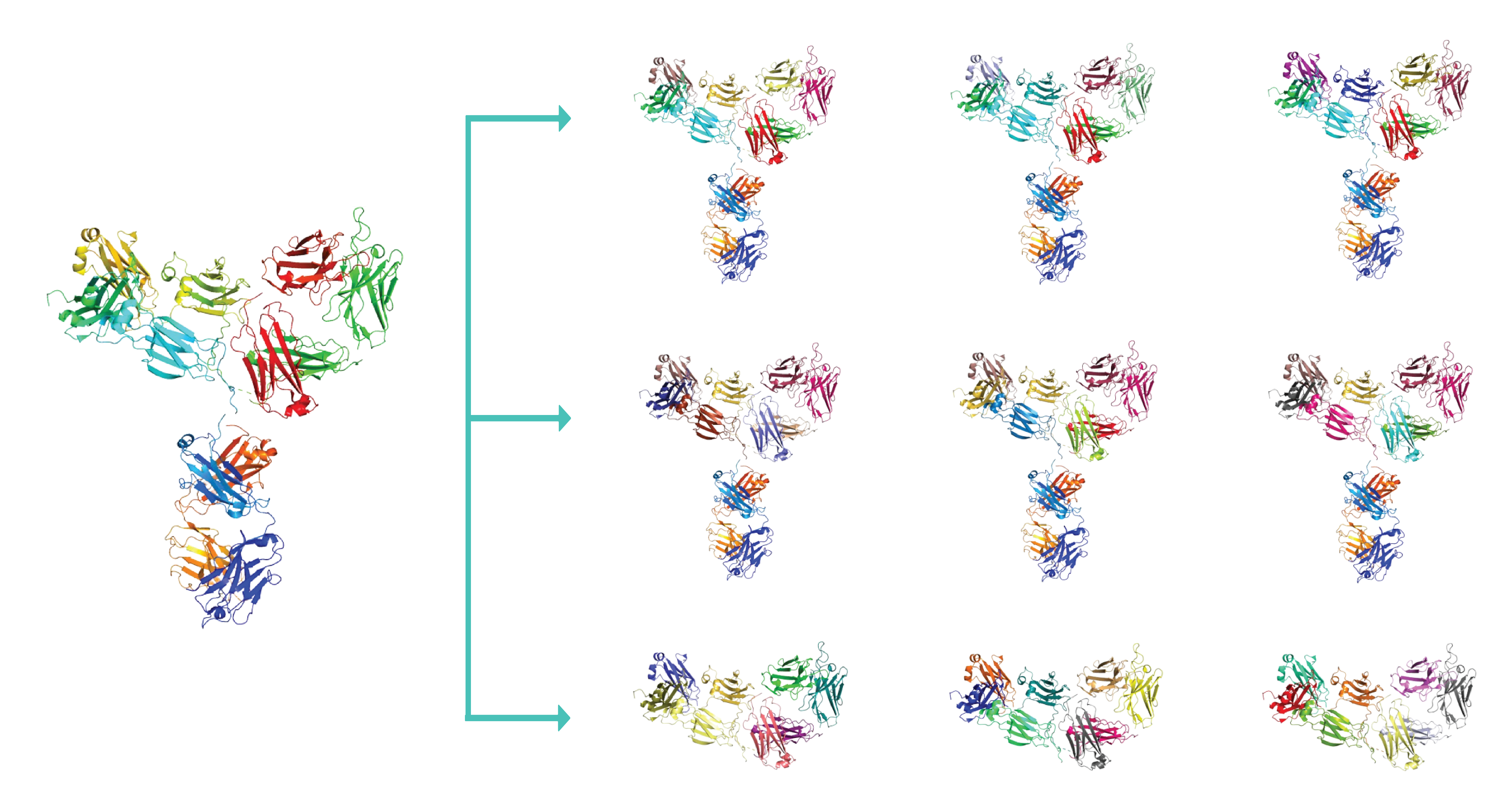
Antibodies are ideal therapeutic scaffolds - highly selective and generally have higher affinities than T-cell receptors. Immuno-oncology therapeutics are currently exploring two similar paradigms for leveraging pHLA complexes – Chimeric Antigen Receptor on T-cells (CAR-T) and Bispecific antibodies which bind to pHLA complexes and T-cell activating proteins such as CD3. By directing T-cell engagement and activation to cancer-specific peptides on the pHLA complex, precision cancer cell kills can be achieved.
 ReD and Ruby employs lambdoid phage display, which demonstrate superior characteristics in:
ReD and Ruby employs lambdoid phage display, which demonstrate superior characteristics in:
- Higher avidity panning of libraries to ensure high diversity clonal capture against pHLA complexes.
- Lossless panning and enrichment of clones to separate affinity and avidity from clonal numbers.
- Speed and Sensitivity, with picomolar dissociation constant antibodies generated within 20 weeks.
- Human antibody backbone, ready for therapeutic engineering and format conversion.
- Libraries compatible with multiple HLA variants for coverage across patient geographies and demographies
CAR-T and Bispecific Antibodies – Utilising pHLA complex for immuno-oncology therapeutics
While the natural engagers of pHLA complexes are T-cells and T-cell receptors, there are many targets for which it is difficult to develop good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity.
Antibodies are ideal therapeutic scaffolds – highly selective and generally have higher affinities than T-cell receptors. Immuno-oncology therapeutics are currently exploring two similar paradigms for leveraging pHLA complexes – Chimeric Antigen Receptor on T-cells (CAR-T) and Bispecific antibodies which bind to pHLA complexes and T-cell activating proteins such as CD3. By directing T-cell engagement and activation to cancer-specific peptides on the pHLA complex, precision cancer cell kills can be achieved.
CAR-T and Bispecific Antibodies – Utilising pHLA complex for immuno-oncology therapeutics
While the natural engagers of pHLA complexes are T-cells and T-cell receptors, there are many targets for which it is difficult to develop good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity.
Antibodies are ideal therapeutic scaffolds – highly selective and generally have higher affinities than T-cell receptors. Immuno-oncology therapeutics are currently exploring two similar paradigms for leveraging pHLA complexes – Chimeric Antigen Receptor on T-cells (CAR-T) and Bispecific antibodies which bind to pHLA complexes and T-cell activating proteins such as CD3. By directing T-cell engagement and activation to cancer-specific peptides on the pHLA complex, precision cancer cell kills can be achieved.

pHLA complex – Sensitive and discrete differentiation between healthy and cancerous cells
As part of natural protein turnover, all proteins get processed by the proteasome into short peptides, which are then processed by the endoplasmic reticulum and loaded onto the major histocompatibility complex (MHC) - human leucocyte antigen (HLA) in humans. The peptide-HLA (pHLA) complex is transported to the cell surface providing intrinsic surveillance of the internal state of cells.
The natural binding partner for a peptide-HLA complex is the T-cell receptor which has to be able to discriminate between peptide-HLA complexes on the basis of specific interactions with the peptide. Thus, cells that are infected by a virus or contain an altered protein can be detected and destroyed by T-cells.
The challenge of targeting peptide-HLA complexes Antibodies are similar to T-cell receptors and possess innate characteristics which make it advantageous for engineering and therapeutics. Developing therapeutic antibodies against pHLA complexes are extremely challenging, requiring consideration and optimisation of hundreds of interdependent characteristics and parameters, with antibodies requiring exquisite singleresidue precision recognition of peptide sequences on the pHLA complex to delineate healthy from cancer states.
CAR-T and Bispecific Antibodies – Utilising pHLA complex for immuno-oncology therapeutics
While the natural engagers of pHLA complexes are T-cells and T-cell receptors, there are many targets for which it is difficult to develop good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity.
Antibodies are ideal therapeutic scaffolds - highly selective and generally have higher affinities than T-cell receptors. Immuno-oncology therapeutics are currently exploring two similar paradigms for leveraging pHLA complexes – Chimeric Antigen Receptor on T-cells (CAR-T) and Bispecific antibodies which bind to pHLA complexes and T-cell activating proteins such as CD3. By directing T-cell engagement and activation to cancer-specific peptides on the pHLA complex, precision cancer cell kills can be achieved.

ReDTM platform and Ruby library – Scalable, Flexible and High Precision Antibodies
Myrio constructed and operates the Retained Display (ReD) platform and Ruby mass diversity human scaffold library (> 300 billion clonal identities). Together these form an antibody engineering system that is designed to generate and develop pHLA discriminating antibodies.

ReD and Ruby employs lambdoid phage display, which demonstrate superior characteristics in:
- Higher avidity panning of libraries to ensure high diversity clonal capture against pHLA complexes.
- Lossless panning and enrichment of clones to separate affinity and avidity from clonal numbers.
- Speed and Sensitivity, with picomolar dissociation constant antibodies generated within 20 weeks.
- Human antibody backbone, ready for therapeutic engineering and format conversion.
- Libraries compatible with multiple HLA variants for coverage across patient geographies and demographies
pHLA complex – Sensitive and discrete differentiation between healthy and cancerous cells
Technology
Being large proteins, antibodies cannot enter cells due to their size. As a result, the current market for antibodies – valued around US$120 billion – is based entirely on targeting cell-surfaces and soluble proteins.
Most of the changes that occur in the transformation of a normal cell into a cancer cell take place behind the cell membrane making them impossible for ‘traditional’ antibodies to be useful.
Every protein inside every nucleated cell will be broken up into short peptides by the proteasome as part of natural protein turnover. These peptides are taken up into the endoplasmic reticulum where some of them are loaded into a carrier molecule called major histocompatibility complex (MHC) also referred to as human leucocyte antigen (HLA) in humans. The peptide-MHC complex is then transported to the cell surface providing a glimpse of what is happening within the cell.

The natural binding partner for a peptide-MHC complex is the T-cell receptor which has to be able to discriminate between peptide-MHC complexes on the basis of specific interactions with the peptide. Thus, cells that are infected by a virus or contain an altered protein can be detected and destroyed by T-cells.
Antibodies, like T-cell receptors, are from a family of proteins called immunoglobulins and both have similar structures consisting of two protein chains that together form the antigen, or target, binding site.
The challenge of targeting peptide-MHC complexes
Finding good antibodies against peptide-MHC complexes is extremely challenging because they are extremely rare – sometimes one in a hundred billion. This is because the antibody has to interact with several amino acids along the length of a short linear peptide to achieve selectivity. Large libraries of different antibodies are required, and it is essential that these rare binders are not lost during the screening process due to loss of diversity.
The Myrio difference
Myrio has designed and built a novel platform around its Retained Display (ReD) technology that is unique in its ability to consistently find quality binders against peptide-MHC complexes. It works because ReD is a ‘lossless’ system i.e. there is no loss of diversity through the discovery process and this is critical when trying to find very rare binders.
ReD is a unique antibody discovery engine that is quite distinct from either the use of transgenic animals or filamentous phage display.
ReD employs a lambdoid phage which has a number of advantages over filamentous phage display. The key points of difference between filamentous phage display and ReD are as follows:
- Higher avidity panning of libraries to ensure capture of all clones that bind the target
- High-speed cell sorting of the many captured clones using clonal cell-display of each binder
- No loss of diversity during the panning and enrichment steps making ReD the first ‘lossless’ discovery platform – essential when looking for very rare binders
- Due to its cell-display step, which employs high speed cell sorting, ReD is also incredibly fast achieving industry-leading speed for antibody discovery
Myrio’s Ruby libraries consist of single chain variable fragments (scFv) built on germ-line human antibody sequences and, therefore, require no humanization. The scFv are very stable and can readily be converted to IgG or other formats.
Myrio is happy to discuss the attributes of the ReD system in more detail with interested parties.

Myrio has demonstrated the utility of its scFv binders in bispecific format (anti-CD3) and in chimeric antigen receptor T-cells (CAR-T). This work has been conducted in-house and with our collaborators of global top institutions across a range of different cancers and targets, including neoantigens, human endogenous retroviruses (HERVs), cancer-testis antigens, and wild-type self-proteins.
T-cells and T-cell receptor-based approaches are increasingly attracting attention due to their ability to target intracellular proteins, although the clinical and manufacturing experience with these modalities is still limited compared to monoclonal antibodies. In addition, there are many targets for which it is difficult to find good T-cell receptors or T-cells. These include the majority of neoantigens (mutated proteins that occur in cancer) and self-proteins, for example PSA or p53. This is because any high avidity T-cell that can target a peptide from a self-protein is deleted in the thymus to prevent auto-immunity which occurs when the immune system attacks normal healthy tissue.
Antibodies are highly selective and generally have higher affinities than T-cell receptors. T-cell receptors can be engineered to increase their affinity although this is a lengthy and uncertain process.
Smaller antibody fragments such as scFv provide the option of manufacturing in microbial systems, such as in E. coli. This enables faster scale-up and a lower cost of manufacturing; a factor that can be important in response to emergent diseases, for example.